The human brain consists of billions of nerve cells that are organized in defined neural networks. They control biological processes such as learning and memory and enable humans to interact with other individuals in a changing environment. The research projects of the Department of Molecular Neurogenetics at the ZMNH focus on the question of how plastic changes become permanent and which synaptic molecules and mechanisms alter synaptic strength and cognition. We investigate individual genes and proteins in neurons and ask which molecular mechanisms stabilize the structure and function of neuronal synapses.
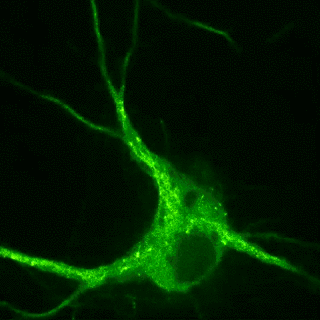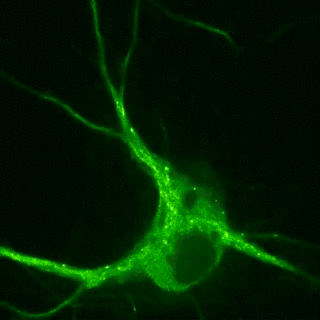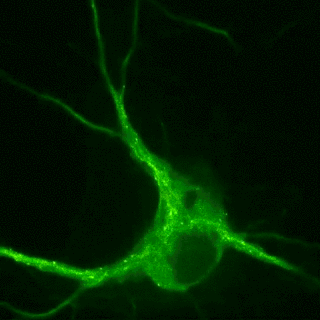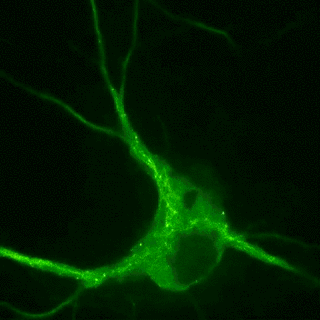
Plasticity-related proteins dynamically enter and leave neuronal synapses in an activity-dependent manner. These trafficking processes require a dynamic actin and microtubule cytoskeleton and employ molecular motors to control the delivery and removal of synaptic components such as postsynaptic neurotransmitter receptors. Although synapses are unlikely to contain a molecular address, the delivery of new material is tightly controlled during plasticity. For example, the combinatorial use of different cargo adaptors or the post-translational modifications of microtubules, the pathways on which the motors move, contribute to transport specificity. Our goal is to understand how cytoskeletal dynamics and the regulation of transport are involved in shifting the dynamic balance of synaptic components in plasticity.

We study learning and memory in genetic mouse models to determine whether and how the loss or dysfunction of individual genes contributes to mammalian behavior. We use reverse genetics to study genes that control exploration, anxiety, motor skills and cognitive performance. An important goal is to place molecular findings at neuronal synapses in the functional context of a complex brain.
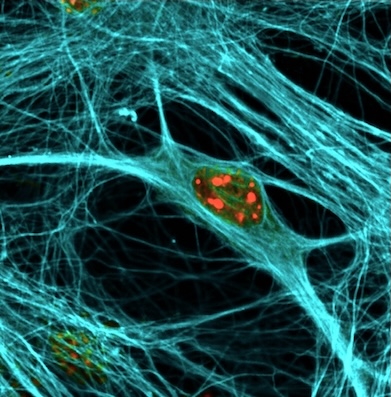
Dysfunctions of various genes that code for synaptic proteins and components of transport are the cause of mental disability, cognitive decline and neurodegeneration. Accordingly, autism spectrum disorders and schizophrenia are referred to as “synaptopathies”. Synaptic dysfunction also occurs in Alzheimer's disease and dementia and is thought to contribute to neuronal loss. In order to identify pathogenic mechanisms that can contribute to the development of drugs and neuroprotective strategies to combat brain diseases and cognitive decline, we are investigating the dynamics of key proteins such as APP, tau and prions in synaptic diseases.